- York House Church Lane, Chalfont St Peter,
Gerrards Cross, Buckinghamshire, SL9 9RE - 666-888-0000
- info@tersanpharm.com
- info@tersanpharm.com
- York House Church Lane, Chalfont St Peter,
Gerrards Cross, Buckinghamshire, SL9 9RE - Contact Us
Oct2018
Tersan’s AI provides evidence for 3 years clinical efficacy of ANAVEX®2-73 Alzheimer’s targeted therapy @CTAD2018
Precision medicine approach using Tersan’s AI KEM® shows that a small data rich open label clinical study of 32 well-characterized patients may be sufficient to identify strong biomarker hypothesis identifying patients with the highest chance of benefiting from the drug.
BARCELONA, October 26th, 2018. Dr Mohammad Afshar presented 3 years longitudinal data of clinical efficacy of ANAVEX®2-73 (Anavex Life Sciences, Nasdaq: AVXL), and confirmed role of patient selection genomic biomarkers, at the 2018 Clinical Trials on Alzheimer’s Disease Meeting (CTAD).
Using Tersan’s proprietary KEM® (Knowledge Extraction and Management) advanced Artificial Intelligence technology, the analysis of the longitudinal 3-year (148-week) data showed that patient cohort with the higher concentration of ANAVEX®2-73 maintains Activities of Daily Living Score (ADCS-ADL) and display a reduced cognitive decline (MMSE) when compared to the lower concentration cohort.
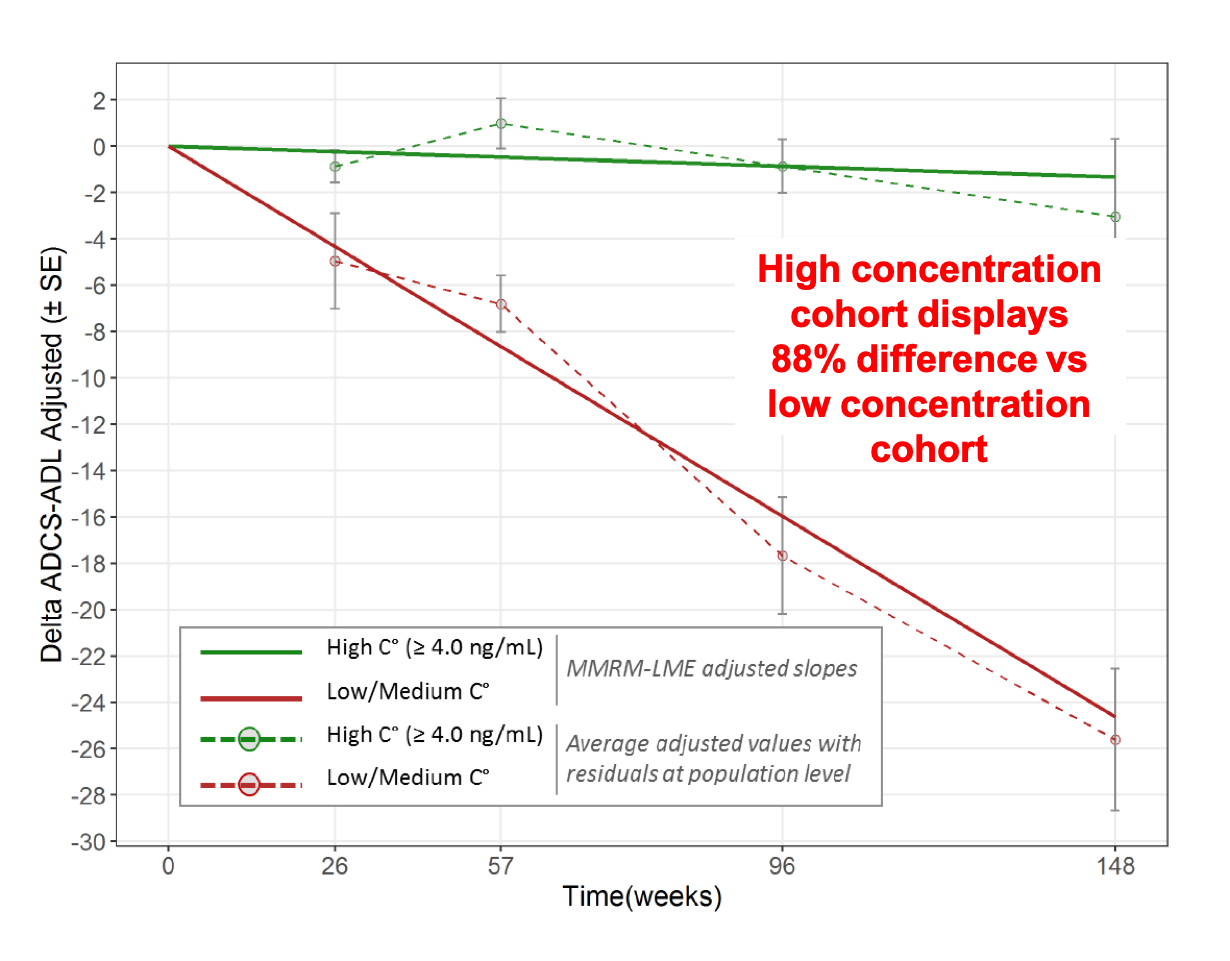
In contrast to the vast majority of communications at CTAD 2018, the presentation focused on a novel drug target (SIGMAR1), the use of genomic biomarkers, rather than surrogates of response, and demonstrated the ability of the precision medicine approach to confirm significant drug response effects. The observed 3-year effect was large: 88% improvement compared to lower dose for ADCS-ADL (p<0.0001) and 64 % less decline for MMSE (p<0.0008).
“We are excited to see our initial biomarker hypothesis confirmed at 148 weeks. The biomarkers were selected through a data driven unbiased systematic analysis of all available genomic and clinical data, identifying a genomic alteration of SIGMAR1, the putative target of ANAVEX 2-73. The consistency of the DNA and RNA data, as well as multiple end-points and time-points further strengthen this biomarker hypothesis.” Mohammad Afshar, MD, PhD, CEO of Tersan Pharmaceuticals Ltd.“This innovative data analytics approach, using our KEM® Artificial Intelligence platform has the potential to expand the access to precision medicine and precision pharmacology for a wide range of neurodegenerative diseases, thus, identifying the right patients that can benefit from the right therapy, at the right moment.” he added.
Given the current gloom from negative results of late stage clinical trials built on the prevailing amyloid paradigm, which have reported non-conclusive results or worse, have demonstrated negative impact, the novel therapy approach taken by Anavex, using a novel target SIGMAR1 and genomic inclusion biomarkers selected using the KEM Artificial Intelligence platform, brings hope to the field.

Further Readings:
Press Release – Anavex Life Sciences Presents New Three-Year, Longitudinal Clinical Efficacy Data for ANAVEX®2-73 in Alzheimer’s Disease at the 2018 Clinical Trials on Alzheimer’s Disease (CTAD) Meeting. (Anavex press release)
2018 CTAD CORPORATE PRESENTATION – Longitudinal 148-Week Update of ANAVEX®2-73 Phase 2a Alzheimer’s Disease Extension Study. (Presentation PDF)
-
Tags:
- alzheimer's, recent post

