Tersan’s XAI Driven Drug Development Platform :
Accelerated Path From Design to Approval
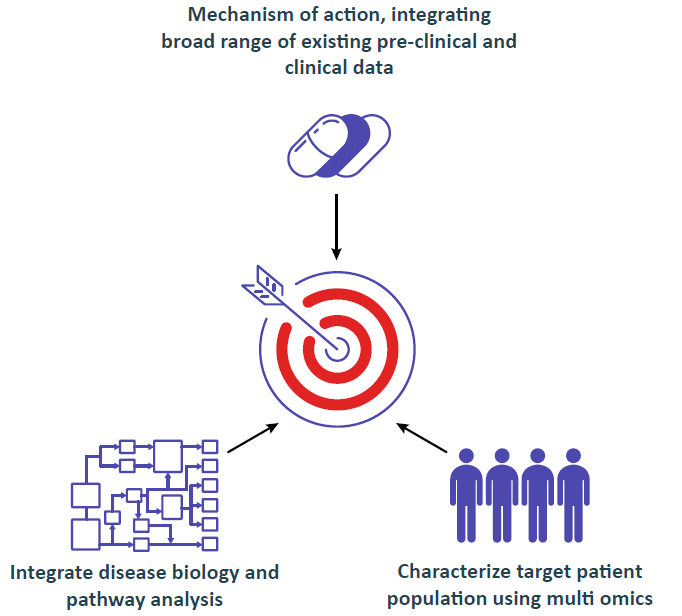
AI Driven Knowledge Integration and Discovery Platform Aiming at Regulatory Strategy

Integrating 100,000s parameters, starting with as few as 10s of patients
Partnerships
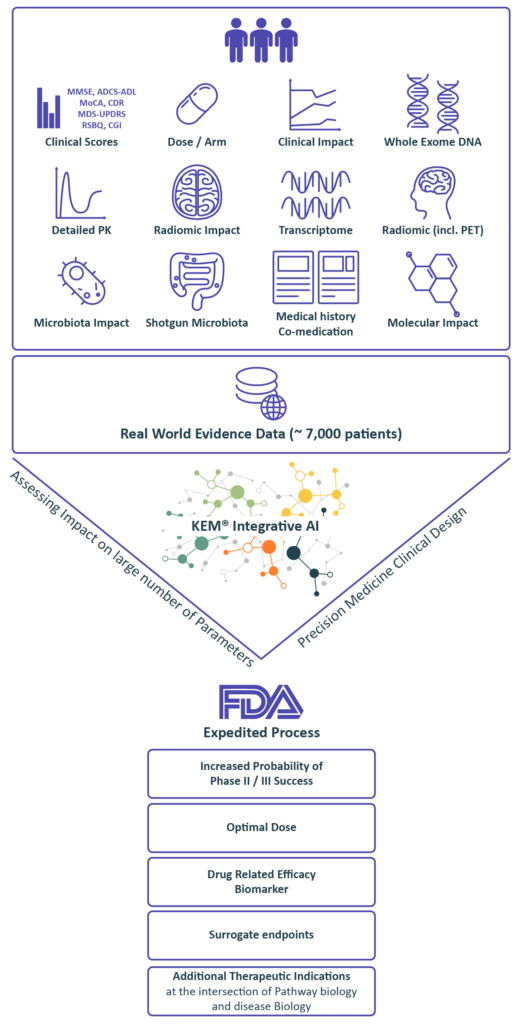
RWE Patients Records
Years of experience
Total Investment
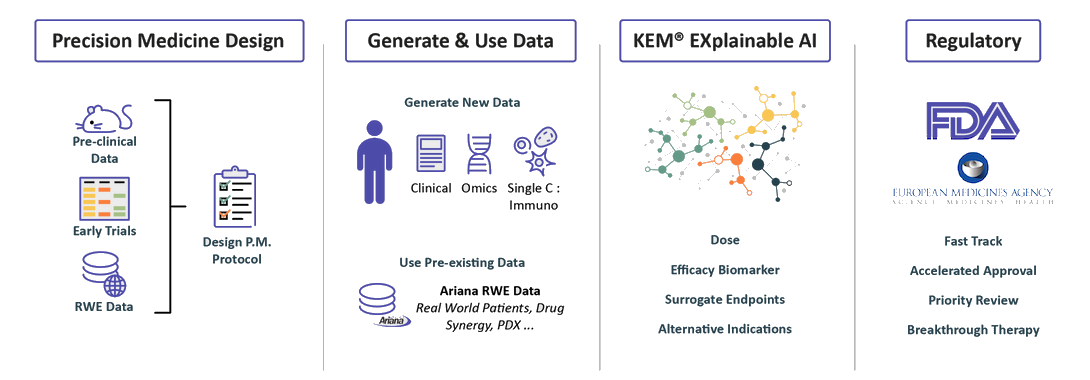
We design Precision Medicine Clinical protocols
We can start our process at the pre-clinical
stage or with as few as 10 patients.
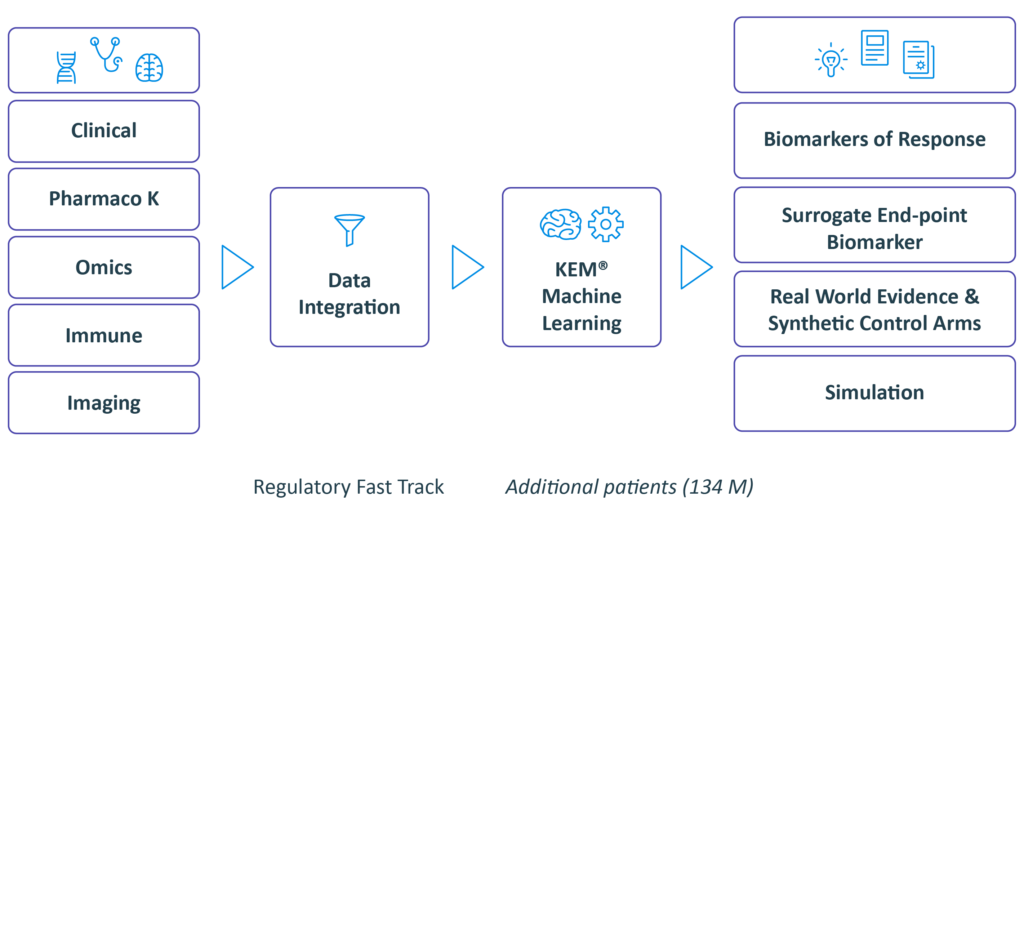
We integrate pre-clinical data, data from
past clinical trials when they exist.
Real World Evidence data from our extensive knowledge base in order to identify biomarkers and design a data enriched clinical protocol.

We collect & generate data

Integrate and discover with our XAI Technology
we identify:
• Optimal dose
• Companion biomarkers and best responders end-points and surrogate endpoints
• Synergistic drug combinations
• Novel indications

Regulatory Strategy

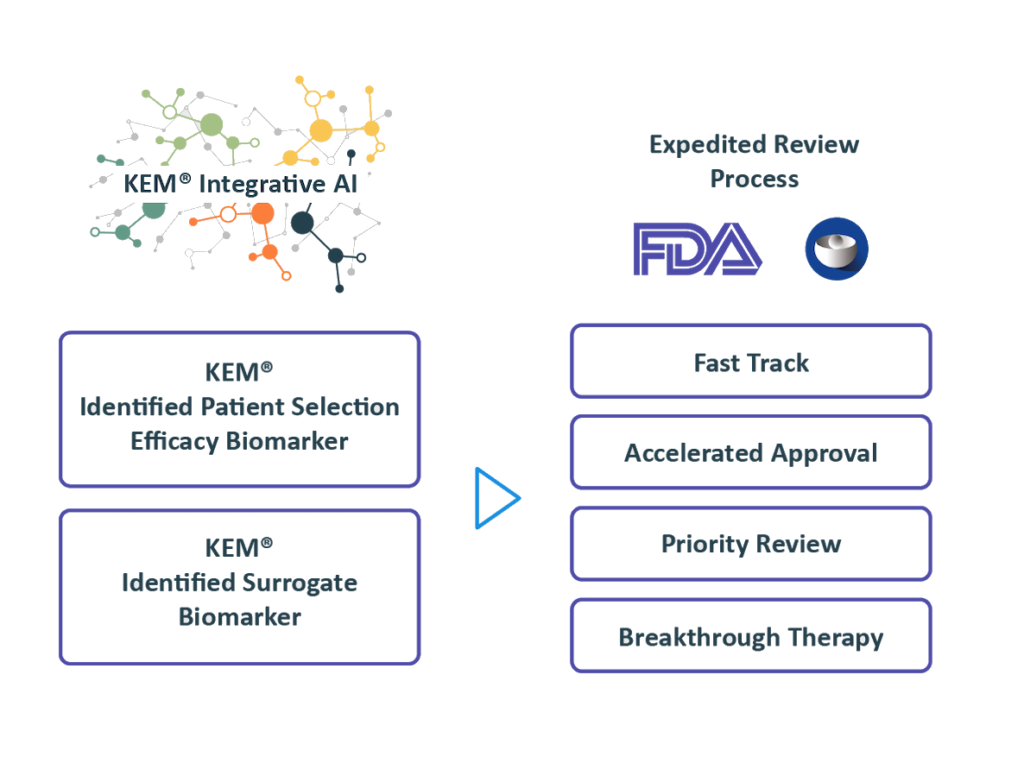
Benefiting from over 10 years of close interactions with the FDA,
we transform findings from the KEM® platform into optimal regulatory strategies for accelerated approval
KEM® Technology validated by a large number of partners


Recents Articles
Latest Press Releases
09May
Tersan Pharmaceuticals Ltd continues to bolster Scientific Advisory Board by adding world-leading Oncologist Pr. Jean-Yves BLAY
Tersan Pharmaceuticals Ltd is a leading Artificial Intelligence (AI) drug developme...
27Mar
Tersan Pharmaceuticals Ltd strengthens SAB with appointment of world-leading Neurologist Jacques Touchon
Cambridge, MA, USA and Paris, France, March 27, 2023 – Tersan Pharmaceuticals Ltd...


30Nov
CY6463 administration is linked to improvements in Alzheimer’s disease-relevant biomarkers, as revealed by eXplainable AI-driven analysis of multiple Phase 1 clinical trials
• Phase 1 studies show improvement in cognitive factors, consistent ...

