Tersan® Real World Data & Evidence (RWD/RWE) platform capabilities
Tersan® has consolidated multiple, publicly available administrative healthcare admissions databases into a locally searchable observational health data resource of patient hospitalization, outpatient and other episodes, comprising in excess of 6B records. This extensive Real World Data & Evidence (RWD/RWE) capability underpins Tersan business services across:
• Precision medicine clinical development (for example, design of data-rich clinical protocols with synthetic control arms).
• Global health impact evidence generation (for example, epidemiological studies of specific diseases or industrial / environmental activities).

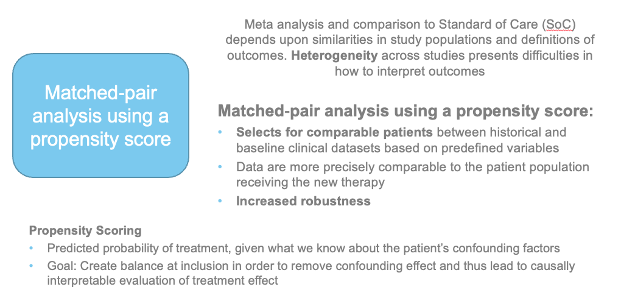
Synthetic control arm for clinical development


•High quality curated electronic health records (EHR) data collected during routine clinical care of patients have the potential to accelerate drug development
•In areas of high unmet need, real world controls could help provide external control for regulatory decision-making
•Requires advanced analytical techniques to address limitations pertaining to RW data (propensity scores)
• « Real world patients » may be different to patients enrolled in clinical trials (with comorbidities, etc.) and use of RW data may provide a more accurate picture of the impact of a novel therapy

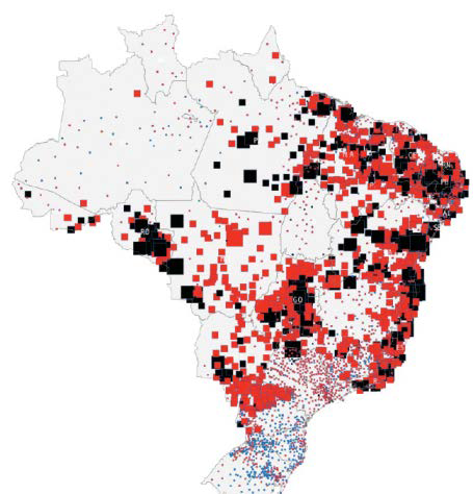
Global Health impact evidence generation
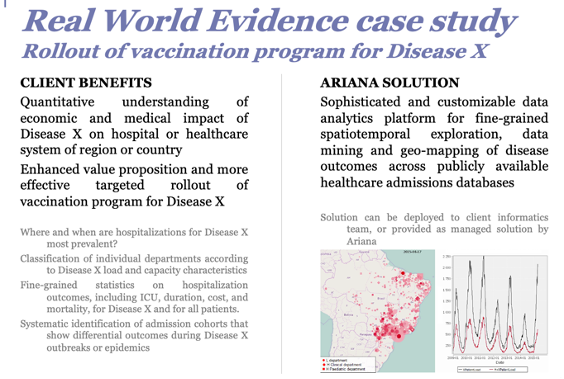
•Tersan has implemented a sophisticated and customizable cloud analytics and reporting platform for evidence generation across these Real World databases:
•“Big Data” epidemiological studies of medico-economic impact of specific diseases or industrial / environmental activities
•Flexible analysis plan execution (including data exploration, data mining and multivariate analysis) over a wide range of spatial and temporal resolutions
•Platform is built using a combination of industry-standard (open) workflow, database, statistical and geo-mapping components, and proprietary data mining algorithms developed by Ariana.
Publication track record in peer-reviewed manuscripts:
•Infectious disease epidemiology for quantification of disease burden, and comorbidity risk factors.

Scientific Publications
Augmenting single-arm Phase 2a open label trials with Real-World external control data.
Afshar et al. 2019 J Prev Alzheimers Dis 6, 45–154.
Mortality among hospitalized dengue patients with comorbidities in Mexico, Brazil.
Macias et al. 2021 AJTMH, 105(1), 102–109.
Real-World Evidence of Dengue Burden on Hospitals in Mexico: insights from the automated...
Macias et al. 2019 RDI Clinica, 71(3), 168–177.
Comorbidities increase in-hospital mortality in dengue patients in Brazil.
Werneck, et al. 2018 MDI Oswaldo Cruz, 113(8), 1–5.

