The authors note that Tersan’s KEM® explainable Artificial Intelligence “platform described in this work opens the possibility of using data-driven unbiased biomarker identification early in the drug development process”, even using small cohorts. “The “white box” and systematic approach (…) is ideal for the analysis of early data, leading to the identification of patient selection biomarkers that can assist in the design of more effective subsequent clinical trials.”
“We believe that the analysis platform described in this work opens the possibility of using big data-driven unbiased genome-wide patient selection marker identification early on in the drug development process of CNS diseases, including Alzheimer’s disease, which is currently applied more routinely in the field of oncology,” said Christopher U. Missling, PhD, President and Chief Executive Officer of Anavex.
KEM® (Knowledge Extraction and Management) is a comprehensive and FDA-tested Explainable Artificial Intelligence (XAI) data analytics platform that enables full exploitation of complex datasets. It has uniquely demonstrated its ability to extract biomarkers and identify alternative indications from small sets of patients, in multiple therapeutic areas including cancer, CNS, metabolic and immunological diseases.
About Tersan Pharmaceuticals Ltd
Tersan Pharmaceuticals Ltd is a leading digital health Company focused on developing advanced therapeutic decision support systems. Using its KEM® Explainable Artificial Intelligence (XAI) technology, Tersan helps its partners introduce personalized medicine clinical trial design into their protocols and identify the best clinical endpoints, the best responders and the best potential synergistic drugs. Tersan routinely collects and combines clinical data with omic data, immunological readouts (such as FACS), microbiota, Patient Reported Outcomes as well as Real World Evidence data. Combining advanced data analytics with regulatory expertise, Tersan helps translate findings into innovative clinical plans. With a growing number of successful therapeutic development applications, KEM® is the only FDA-reviewed technology that systematically explores combinations of biomarkers, producing more effective biomarker signatures for precision medicine. Tersan has developed Onco KEM®, the most advanced, clinically tested, oncology treatment selection system. Founded in 2003 as a spin-off of the Institut Pasteur, Paris, France, the company operates a subsidiary in the United Kingdom since 2012. Further information is available at www.tersanpharm.com.
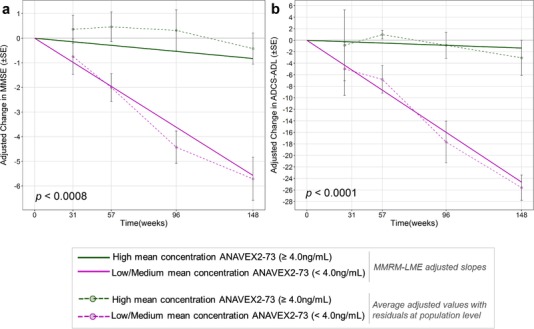
Link to publication : “A precision medicine framework using Artificial Intelligence for the identification and confirmation of genomic biomarkers of response to an Alzheimer’s disease therapy: Analysis of the Blarcamesine (ANAVEX2-73) Phase 2a clinical study.“